HLA-G: from pregnancy to tumors
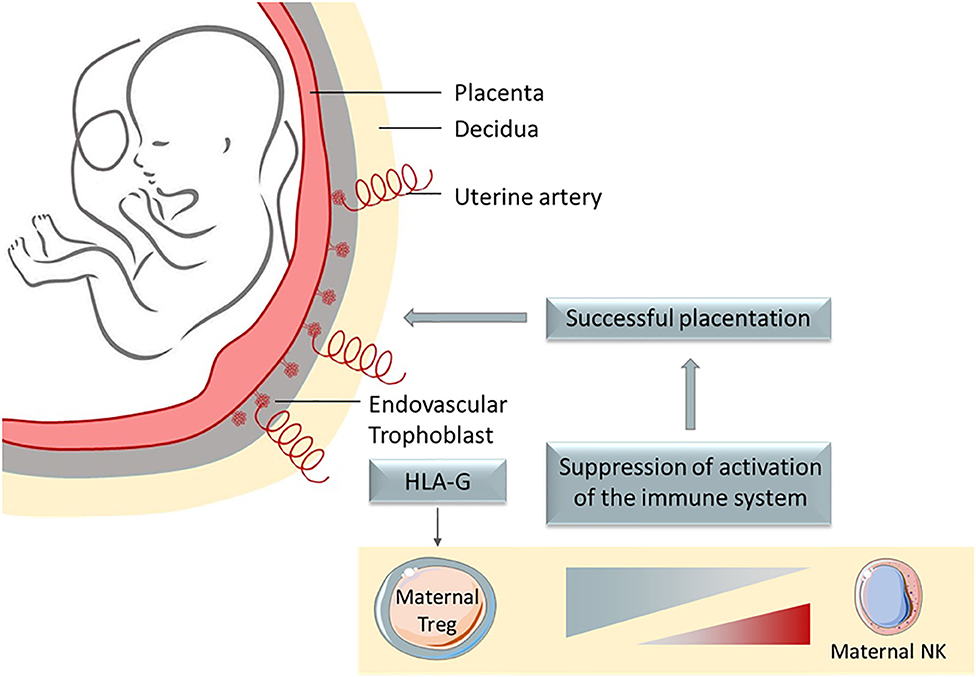
Invectys Inc. targets a powerful immune checkpoint named HLA-G. HLA-G is a non-classical MHC class I molecule normally only expressed at the fetal-maternal interface, where it protects the semi-allogenic fetus from the maternal immune system. Otherwise, the father’s antigens would be recognized as foreign and thus destroyed. To avoid this, HLA-G serves as a broad-range immune checkpoint which shuts down any immune response towards the fetus. HLA-G expression on the placenta not only inhibit all immune cells but also creates a suppressive micro-environment in which the immune system is repressed, thus protecting surrounding cells as well.
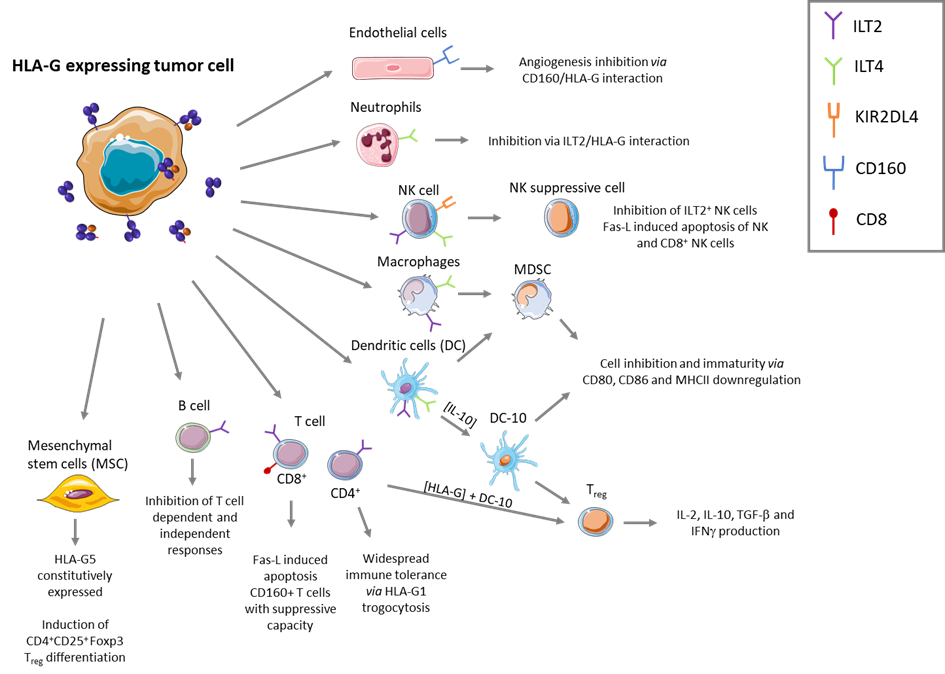
HLA-G interacts with 2 main inhibitory receptors: ILT2 and ILT4. ILT2 is expressed on all immune cell subsets whereas ILT4 is only expressed on myeloid cells (cells of the native immune system). The HLA-G/ILT2 interaction inhibits T cells function, protecting cells from the direct cytotoxic of CD8+ cells. Conversely, the HLA-G/ILT4 interaction induces inhibition of myeloid cells maturation and turns them into suppressive Antigen Presenting Cells (APCs), setting up the immuno-suppressive TME. Thus, HLA-G provides protection both directly and indirectly.
HLA-G expression is restricted to immune-privileged sites in healthy individuals. Yet, given its broad immunosuppressive action, tumors neo-express HLA-G to evade immune response. Indeed, more than 70% of cancers use HLA-G as a shield to disrupt the patient’s immune system’s ability to target the tumor cells. As such, HLA-G is defined as tumor specific antigen (TSA) since it is barely expressed on healthy tissues but neo-expressed on tumors implying that HLA-G represents a critical – although complex – target for immunotherapy.
Invectys takes advantage of this widely prevalent target through two different technologies: CAR-T cells and antibodies.
IVS-3001 – Destroying HLA-G-bearing cancer cells: anti-HLA-G CAR-T cells
Invectys has developed a 3rd-generation CAR construct allowing anti-HLA-G CAR-T cells to target and kill HLA-G-bearing cells, resulting in our lead CAR-T cell: IVS-3001. These are the first in class CAR-T cells engineered to target the immune checkpoint HLA-G.
Our CAR-T cells are currently under late stage of preclinical development and were granted a $14.2 million CPRIT funding by the State of Texas to initiate clinical trials in 2022 against advanced carcinoma.
Anti-HLA-G CAR-T cells have demonstrated in vivo their ability to migrate to tumor sites and destroy HLA-G-bearing cells. Mice treated with anti-HLA-G CAR-T cells have shown a near-total absence of tumor growth.
This approach has several unique benefits:
- IVS-3001 is not hindered by the immuno-suppressive effects related to the HLA-G interaction with the ILT2 receptor which could be expressed by T cells.
- Since HLA-G expressing tumor cells were shown to induce suppressive APC, regulatory T cells and suppressive NK cells displaying HLA-G on their surface, by eliminating HLA-G+ cells, Invectys’ CAR-T cells disrupt the HLA-G related immuno-suppressive microenvironment created by the tumor, allowing the restoration of the patient’s immune response.
- HLA-G represents the best tumor specific antigen to date since it is almost not expressed on healthy tissues. Adverse events related the “on-target, off-tumor” effects will be very limited if not absent.
- IVS-3001 covers the main immune-suppressive HLA-G isoform expressed by carcinoma
As a result, Invectys’ CAR-T cell projects were awarded by multiple independent organisms: Cancer Prevention Research Institute of Texas award 2020, “Best Project” award at the MATWIN 2019 competition, CAR-T poster selected for top 10 at ESMO Immuno Oncology, Geneva, December 2019, and Awarded by the EU-funded (France, Germany, Italy & United Kingdom) collaborative research project CARAT (Chimeric Antigen Receptors for Advanced Therapies).
A glossary is available for technical definitions.